The mesothelin (MSLN) protein is overexpressed in many solid tumors and is considered a viable immunotherapeutic target. We recently demonstrated that MSLN is aberrantly expressed on the blast surface in approximately 1/3 of AML cases and lacks expression in normal hematopoiesis, making it an attractive target in AML. While the exact function of MLSN is unknown, multiple studies, including its recently described role in the development of surgical adhesions, implicate it in cell adhesion in both healthy and malignant cells. We hypothesized that MLSN expression in AML may be involved in extramedulllary disease (EMD). We correlated MLSN transcription expression with EMD status and utilized xenograft models to evaluate MSLN's role in AML in vivo.
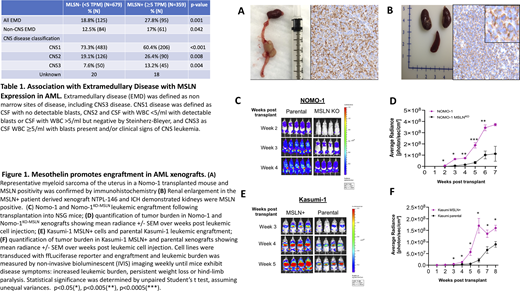
A total of 1,038 patients with de novo AML (age 0-29 years) enrolled on COG AAML1031 with available transcriptome, clinical, and biologic data were included for analysis. Transcriptome sequencing was performed on diagnostic bone marrow or peripheral blood specimens and gene expression was quantified as transcripts per million (TPM). The cohort with MLSN positive (MSLN+) AML (n=359) was defined as patients with MSLN overexpression, 5 TPM, and <5 TPM was considered MSLN-negative (MSLN-; n=679). EMD was defined as having non-bone marrow sites of disease and/or CNS positive (CNS3) disease (CSF WBC 5/ml with blasts or clinical signs of CNS leukemia). Xenograft experiments were performed in NSG mice using cell lines (CDX) or patient leukemias (PDX). Parental Nomo-1, which express endogenous MSLN, and Kasumi-1 (MSLN-) and their engineered Nomo-1 MSLN knockout (Nomo-1KO-MSLN) or MSLN-transduced (Kasumi-1-MSLN+) counterparts and we developed the MSLN+ PDX NTPL-146 for use in CDX and PDX studies respectively. Engraftment was measured using non-invasive bioluminescent (IVIS) imaging.
Our analysis demonstrated a striking association between MSLN expression and EMD. Among the MSLN+ cohort, the rate of EMD was significantly higher compared to the MSLN- cohort (27.8% vs. 18.8%, p=0.001; Table 1). When stratified by EMD type, we observed a similar pattern with higher rates of both non-CNS and CNS EMD in MSLN+ AML. Non-CNS EMD occurred in 17% of MSLN+ vs. 12.4% of MSLN- AML (p=0.042). The rate of CNS positive (CNS3) disease was significantly higher in the MSLN+ cohort compared to the MSLN- cohort (13.2% vs. 7.6%, p=0.004; Table 1). While MSLN+ cases made up 35% of the cohort, they accounted for 47% of the CNS positive cases. Of interest, MSLN+ disease was also associated with significantly higher occurrence of CNS2 disease compared to MSLN- (26.4% vs. 19.1% respectively, p=0.008; Table 1). In patients with very high MSLN expression (100 TPM, n=112), 29% (n=32) of patients had EMD (n=23 non-CNS, n=9 CNS). Notably, among the cohort in this very high expressing group that lacked EMD at diagnosis but who experienced relapse (n=35), 38% (n=13) had EMD at relapse. Among patients with relapsed/refractory (R/R) AML (n=486), MSLN overexpression was also highly associated with EMD. Although MSLN+ cases comprised 35% of R/R cases, they accounted for 53% of EMD cases. The rate of EMD was 40.8% in MSLN+ cases vs. 18.9% of MSLN- cases (p<0.001).
We evaluated the role of MLSN in EMD and its potential function in engraftment in vivo with xenograft models. A higher incidence of EMD was detected in MSLN+ xenografts; 90% (9/10) of Nomo-1 xenografts developed abdominal myeloid sarcomas compared to 30% of Nomo-1KO-MSLN (p<0.005; Fig 1A). The MSLN+ PDX showed EMD and associated renal sarcomas and enlargement of the kidneys (Fig 1B). Evaluation of MSLN+ CDX demonstrated more rapid and higher levels of tumor burden compared to their MSLN- counterparts. At 6 weeks post injection, tumor load of Nomo-1 CDX was 3-fold higher than Nomo-1KO-MSLN (p<0.005; Fig 1C,D) and was 8-fold higher in Kasumi-1-MSLN+ compared to parental Kasumi-1 (p<0.05; Fig 1E,F).
Our findings demonstrate that MSLN is highly implicated in EMD and our xenograft studies further support the hypothesis that MSLN may be involved in cell adhesion and establishing sites of disease. EMD can be very challenging to treat, and in the setting of recurrence often indicates incurable disease, thus novel therapeutic strategies for this group of patients are urgently needed. Our findings support additional studies of how MSLN may be functionally implicated in the development of EMD as well as clinical trials that evaluate MSLN targeting in AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal